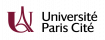The research activities of the Radiation and Living (REV) team are part of this thematic context and have three unifying objectives:
- to develop new experimental and methodological approaches to better understand the effects of ionizing radiation on living organisms;
- to improve the control of the dose deposited in external and internal radiotherapy
- and to reinforce the efficiency of treatment methods in molecular radiotherapy through the production of new radionuclides, the development of new chelators to better target the tissues to be treated or imaged, and the implementation of more precise individualized dosimetry protocols.
These objectives can divided down into three main areas:
1) Study of the effect of ionizing radiation from the cellular to the tissue level: projects “Effects of radiation on tumor growth” and “Studies of the RBE/LET relationships”.
The project “Effects of radiation on tumor growth” aims at studying in a systematic and comparative way the lethal or sub-lethal sub-cellular metabolic effects as well as the macroscopic biological effects on cell proliferation, migration and morphology induced by different types of radiation (X, gamma, H, C, O, Ne) and different irradiation modalities (dose fractionation, radiosensitizing nanoparticles). The analysis methods used include cell biology (culture and growth monitoring), biochemistry (detection of metabolic parameters), optical techniques (epifluorescence videomicroscopy for tumor growth data and IR spectroscopy for analysis of macromolecular modifications) and animal experimentation (study of tumor growth). One of the objectives is more particularly to study the effect of interactions between healthy and irradiated cells on tumor growth (bystander effect). This work is carried out in collaboration with the Modeling of living (MoV) team.
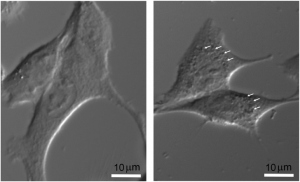
Internalization of radiosensitizing nanoparticles in F98 murine glioma cells, and monitoring of cell viability as a function of X-ray dose with or without nanoparticles (Gd).
Collaborations: Institut Curie (doses, irradiation, …), Nanobactérie SARL (nanoparticles and CIFRE funding), ALBA synchrotron (Barcelona)
2) Multiscale dosimetry for the control of internal radiotherapy: THIDOS project
The THIDOS project aims at reinforcing the control of the delivered dose during the treatment of thyroid diseases with radioactive iodine by reducing the uncertainties related to the calculation of the doses. This project is mainly based on the development of a high spatial resolution ambulatory gamma camera specifically designed to improve the quantitative measurement of the biokinetics of Iodine 131 in the thyroid and organs at risk before and after treatment administration. The second axis, led by the RSN, focuses on the analysis of the reliability and quality of dosimetric calculations based on the integration of different clinical data (functional, anatomical or pharmacokinetic), through the implementation of innovative error propagation methods such as bayesian networks to estimate dose uncertainties. The THIDOS project is funded by the Cancer Plan (AAP Physicancer, INSERM).
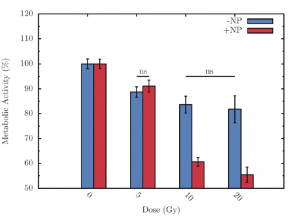
Prototype of a mobile gamma camera for the quantitative measurement of the dose to the organs during radiotherapy.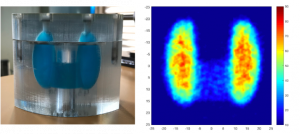
Measurement of the 131I dose using the prototype gamma camera on a thyroid phantom.
Collaborations: Internal Dose Assessment Laboratory (LEDI-IRSN) (dosimetry), Claudius Régaud Institute (University Cancer Institute of Toulouse Oncopole) (clinical evaluation), National Centre of Microelectronics (CNM-CSIC) (microelectronics), Nuclear Physics Institute (Prague) and Univ. Santiago de Compostela (dosimetry), Institute for Corpuscular Physics (IFIC, Valencia) (electronics) and European Radiation Dosimetry Group (associate members).
3) Tools for the production and implementation of new radioisotopes for internal radiotherapy: PRISM project
The need for medical radioisotopes is currently significant, particularly in the field of cancer pathologies. The PRISM project proposes to produce new radionuclides that are difficult to produce at present, through the development of a methodology to ensure their availability in sufficient quantity and quality (chemical and isotopic purity) in order to promote their pre-clinical evaluation. It is particularly interested in Terbium isotopes which allow a theranostic approach, coupling PET imaging (152Tb, 155Tb) and alpha or beta therapy (149Tb, 161Tb), with the same radiopharmaceutical support (chelator-vector set). The objectives are to remove two obstacles by proposing, on the one hand, to use high purity 155Gd produced by SIDONIE (a separator unique in Europe for its separation qualities) to carry out the 155purGd(p,n) to 155Tb reaction (by demonstrating the possibility of producing 149Tb, 152Tb and 155Tb concomitantly with SIDONIE) and, on the other hand, to optimize an original chelator which, conjugated with monoclonal antibodies, will allow to be radiolabeled to Tb under non-denaturing conditions of the biological vector.

SCALP/SIDONIE device for isotope production and separation (IJC Lab).
Collaborations: Subatech (Nantes), ARRONAX (St Herblain), ICMUB (Institut de Chimie Moléculaire de l’Université de Bourgogne, Dijon), IC-UNISTRA (Institut de Chimie de Strasbourg), GANIL (Caen), ILL (Grenoble)